Boyle’s Law (Constant Temperature) is one of the three special cases of Ideal Gas Law. The other two are Charles Law (Constant Pressure) and Gay-Lussac Law (Constant Volume).
In fact, when Boyle’s Gas Law is substituted with Charles Law and Gay-Lussac Law, develops into Combined Gas Law. Moreover, when further combined with Avogadro’s Law yields Ideal Gas Law.
What is Boyle’s Law Of Thermodynamics?
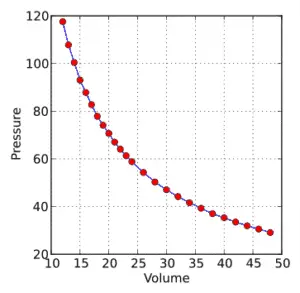
Boyle’s Law is an Ideal Gas Law that basically defines that how the pressure of a given mass of a gas is inversely proportional to the volume of the given gas (or vice-versa) at a constant temperature within a closed system.
In fact, like all the other Ideal Gas Law, Boyles Law describes the behavior of an Ideal Gas. Yet, this pressure-volume law can also be applied to real gases at normal temperatures and low pressure.
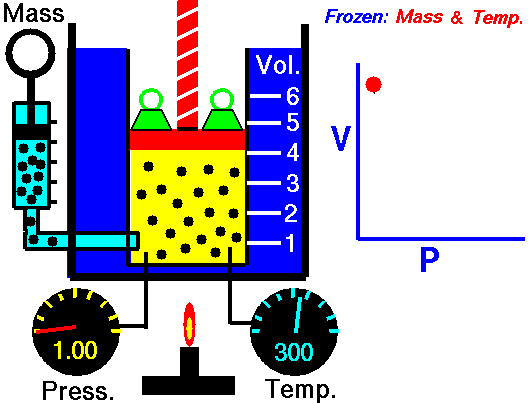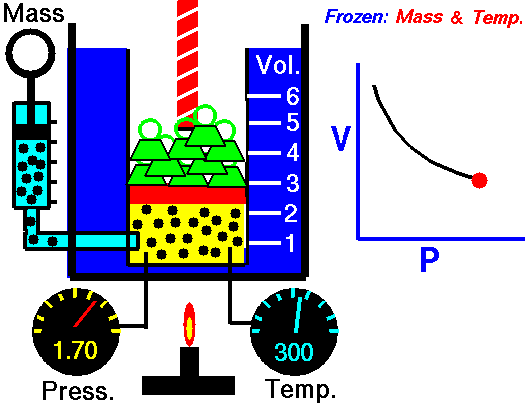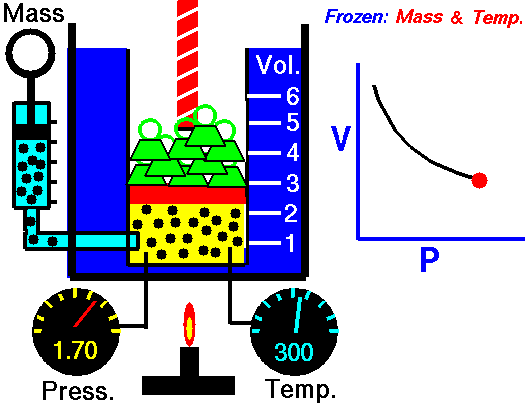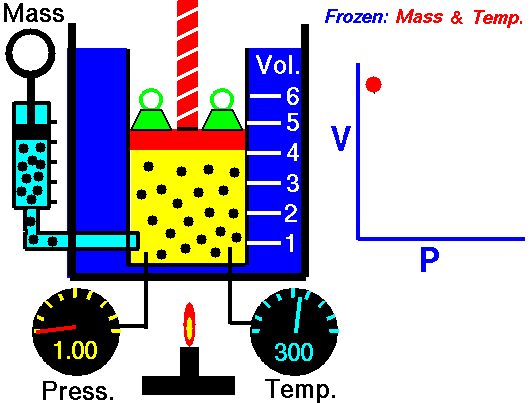
In other words, at a constant temperature, the volume of a given mass of a gas is inversely proportional to its pressure. This law is known as Boyle’s Law.
Boyles Law was named after the Anglo-Irish Chemist, Inventor, and Physicist Robert Boyle who first proposed the original law in 1662. Boyle’s law can be deduced from the Kinetic Molecular Theory Of Gas.
On the other hand, this relationship between pressure and volume was the first physical law expressed in the form of an equation that described the dependence of two variable quantities.
Check out, Sublimation Definition, Process, Facts & Examples
Historical View of Boyles Law
Before Robert Boyle, in the early 17th century; this pressure and volume relationship was first observed by two English Physicists Richard Townley and Henry Power. That’s why Boyle named this gas law as Mr. Townley Hypothesis.
Related, Top 6 Applications Of Boyle’s Law
Apart from this, there are so many controversies over Robert Boyle’s Law. Well, some authorities argue that it was Robert Boyle’s assistant (Robert Hooke) who experimentally discovered the relationship between pressure and volume, not Robert Boyle.
While performing an experiment, Robert used to pour mercury into a j-shaped closed tube. After pouring mercury into the tube, he used to force the air on the other side of the tube to contract the pressure of mercury.
Every time he did an experiment, he used a different amount of mercury every time. Finally, after measuring the experimental data; he discovered that under a certain controlled condition, “in a closed system, the pressure of the gas is inversely proportional to the volume occupied by the gas“.
Take a look at Top 6 Exclusive Real-Life Examples of Sublimation
Later, in the year 1679, a French Physicist and Priest Edme Mariotte independently discovered the same law. That is why Boyles Law is sometimes referred to as Mariotte’s law or Boyle–Mariotte law.
Almost after two centuries, Boyles Law was mathematically proved by James Clerk Maxwell and later by Ludwig Boltzmann.
Boyles Law Formula
Mathematically, Boyle’s Law is expressed as:
or
where,
P = pressure of the gas
V = volume of the gas
k = Boyles law constant
If we have to compare the same substance under two different conditions, then Boyles law equation can be stated as:
The above Boyles law equation shows that when pressure increases, the volume of the gas decreases in proportion.
Boyle’s Law Example Problem
where,
V1= 5 Litre
p2 = ?





This really answered my problem, thank you!
Thnx. Keep visiting us!!!
Thank you for your great post! It has been very helpful.
thanx, keep visiting us…!!!
I and my neighbor were just preparing to do a little research about this. We got a grab a book from our area library but I think I learned more from this post. I’m very glad to see such great information being shared freely out there.
I am happy you like it, keep visiting us!!!