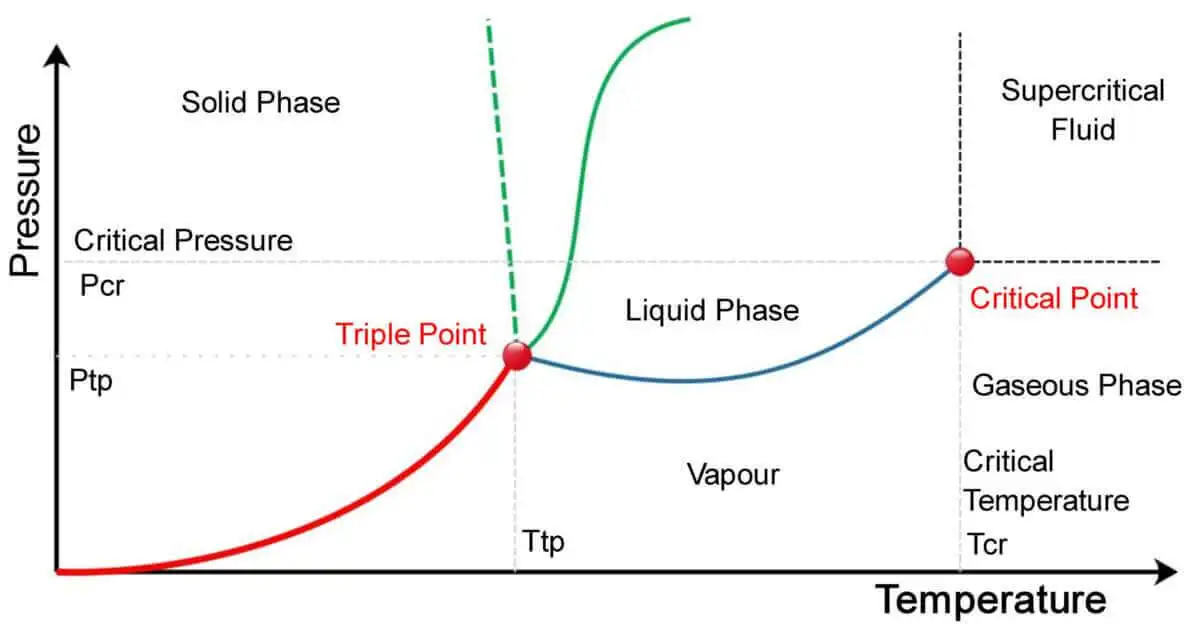
The key difference between triple point and critical point is that the triple point is the temperature and pressure at which a substance coexists in all three phases – solid, liquid, and gas simultaneously. In contrast, the critical point is the temperature and pressure at which a substance undergoes a phase transition from a distinct gas-liquid state to a supercritical fluid state.
In the realm of thermodynamics and phase transitions, understanding the differences between the triple point and the critical point is essential. These two points, with their unique characteristics and applications, contribute significantly to the study of matter and its behavior under different conditions. Therefore, without any more time, let’s dive right in…!!!
Triple Point vs Critical Point
| Aspect | Triple Point | Critical Point | |
| 1. | Definition | The triple point is the unique temperature and pressure at which a substance coexists in all three phases – solid, liquid, and gas simultaneously. | The critical point is the temperature and pressure at which a substance undergoes a phase transition from a distinct gas-liquid state to a supercritical fluid state. |
| 2. | Phase Coexistence | Coexistence of solid, liquid, and gas phases. | The transition between the gas and supercritical fluid phases. |
| 3. | Occurrence | Rarely encountered in nature; achieved under precise conditions. | More commonly encountered in real-world scenarios, especially in chemical engineering and industry. |
| 4. | Conditions | Fixed temperature and pressure. | Fixed temperature and pressure. |
| 5. | Pressure Dependence | Independent of pressure; only temperature-dependent. | Highly pressure-dependent; at a specific critical pressure. |
| 6. | Temperature Dependence | Temperature-dependent; pressure remains constant. | Temperature-dependent; pressure remains constant. |
| 7. | Graphical Representation | Shown as a point on a phase diagram. | Represented as a point on a phase diagram. |
| 8 | Observability | Difficult to observe due to the extreme precision required. | Relatively easier to observe and reproduce in laboratory settings. |
| 9. | Critical Constants | N/A | Critical temperature, critical pressure, and critical density are crucial constants for characterizing the critical point. |
| 10. | Applications | Primarily used for calibrating instruments and defining temperature scales. | Used in various industrial processes, such as supercritical fluid extraction and chemical synthesis. |
Detailed Explanation of 10 Differences Between Triple Point and Critical Point:
- Definition: The fundamental difference between the two points lies in their definitions. The triple point represents a unique state where all three phases of a substance coexist, while the critical point marks the transition from a gas-liquid state to a supercritical fluid state.
- Phase Coexistence: At the triple point, solid, liquid, and gas phases coexist simultaneously. In contrast, the critical point is associated with a transition between gas and supercritical fluid states.
- Occurrence: Triple points are rarely encountered naturally and require precise conditions for their creation. The critical point, on the other hand, is more common and can be found in various real-world applications.
- Conditions: Both triple and critical points have fixed temperature and pressure conditions.
- Pressure Dependence: The triple point is pressure-independent, as it remains constant. In contrast, the critical point is highly pressure-dependent and occurs at a specific critical pressure.
- Temperature Dependence: Both points are temperature-dependent, with pressure remaining constant.
- Graphical Representation: On a phase diagram, the triple point and critical point are represented as single points.
- Observability: The triple point is challenging to observe due to the extreme precision required, whereas the critical point is relatively easier to observe and reproduce in laboratory settings.
- Critical Constants: The critical point is characterized by specific critical constants, including critical temperature, critical pressure, and critical density, which are not applicable to the triple point.
- Applications: Triple points are primarily used for calibrating instruments and defining temperature scales. The critical point finds applications in various industrial processes, such as supercritical fluid extraction and chemical synthesis.
Frequently Asked Questions
1. Why is the triple point important?
Ans: The triple point provides a defined and reproducible standard for temperature and pressure, making it crucial for calibrating thermometers and pressure gauges.
2. Can different substances have different triple points?
Ans: Yes, each substance has its own specific triple point, determined by its unique phase diagram.
3. How is the triple point determined experimentally?
Ans: The triple point is typically determined by carefully controlling the temperature and pressure conditions in a controlled environment, and observing when all three phases coexist.
4. What is the significance of the triple point in thermodynamics?
Ans: The triple point is used as a reference point in thermodynamics to define the Kelvin temperature scale.
5. Is it possible for a substance to have more than one triple point?
Ans: No, under normal conditions, a pure substance has only one set of conditions at which its triple point occurs.
6. Why is the critical point important in chemistry and physics?
Ans: The critical point marks the end of the liquid-gas phase boundary and has important implications for various processes, including phase transitions and chemical reactions.
7. What are some properties of a substance at its critical point?
Ans: At the critical point, the substance has the same density in both the liquid and gas phases, and its properties, such as density and compressibility, become discontinuous.
8. How is the critical point different from the triple point?
Ans: The triple point is where all three phases (solid, liquid, gas) coexist, while the critical point is where the distinction between the liquid and gas phases disappears.
9. Can different substances have different critical points?
Ans: Yes, different substances have unique critical points, which are determined by their molecular properties and intermolecular forces.
10. What is the critical temperature and critical pressure of a substance?
Ans: The critical temperature is the highest temperature at which a substance can exist in a distinct liquid and gas phase, and the critical pressure is the pressure at that temperature.
That’s it for this post. If you like this article, share it if you like, like it if you share it. You can also find us on Mix, Twitter, Pinterest, and Facebook. Hey man, If you have come this far, do give us feedback in the comment section. It would make my day. You can also make a donation. Your donations will help us to run our website and serve you BETTER. Cheers!!!