Each material has a different composition. There is no doubt about it. But, still, all of them have to fall under two categories. In other words, a material can either be a conductor or an insulator. In this exclusive article, I am only going to explain the definition of conductors.
However, I will still clear some air regarding the basic difference between conductors and insulators. See, the materials that conduct electricity are conductors. On the other hand, materials that do not conduct electricity are insulators.
What is Conductor in Physics?
According to the definition of conductors, materials that allow electric charge or electricity to easily pass through them are known as conductors. In other words, an electric conductor allows the electrons to pass from one atom to another.
Not to mention, they also allow heat and light to pass through themselves. Additionally, materials that are primarily made of metals are the best conductors of electricity.
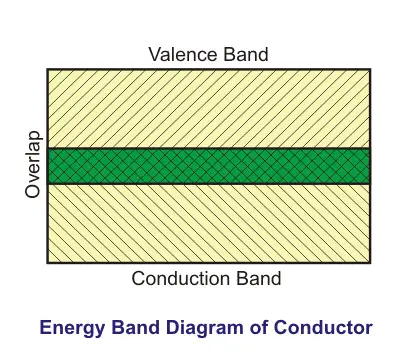
Moving ahead, in a typical conductor, free electrons can move freely anywhere inside a conducting material. WHY? Because there is an overlapping between the valence band and conduction band of the material.
As a result of this overlapping, there is no forbidden gap between the layers of the atomic structure of the conductors. Therefore, when we apply a potential difference across the conductor, due to high conductivity and of course, low resistance, electrons can freely move inside the conductors.
Related: What are Insulators? – Definition, Types & Examples
Facts about Conductors
Did you know that silver is by far the most conductive material available on Earth? Still, it is not widely used as Copper or Aluminium. WHY??
Because silver is far more expensive than copper or aluminum. Therefore, due to its high cost, there are no applications of silver in our household appliances.
However, just because silver is 6% more conductive than copper, scientists use them in making high-tech pieces of equipment such as satellites.
Properties of Conductors
There are so many properties of conductors. However, at the equilibrium condition, an electrical conductor shows the following properties.
- They have low resistance and high conductivity.
- The electric field inside both conductors and insulators is zero.
- Covalent bonds are weak, therefore, can be easily broken.
- The resistivity of conductors can vary from low to high.
- The temperature coefficient of resistance of the conductor is always positive.
- Last, the charge density inside a conductor is always zero, etc.
Highly Recommended: What is Laser Diode? Construction, Types, Working & Applications
Types of Conductors
There are so many different ways to define the different types of conductors. Some of them are explained below. Let’s dive right in!
Based on Ohmic Response of Material
Based on the fact that whether a conductor is following Ohm’s Law or not, conductors can be bifurcated into two types:-
Ohmic Conductors
According to the definition of conductors, conductors that follows Ohm’s law are Ohmic Conductors. Examples of Ohmic conductors are Copper, Aluminium, etc.
Must Read: Alternating Current (AC) vs Direct Current (DC) in Tabular Form
Non-Ohmic Conductors
Conductors that do not follow ohms law are Non-Ohmic conductors. Examples of non-ohmic conductors are a Zener diode, a filament of the bulb, etc.
Based on the Resistivity of Material
The resistivity of the conductor can vary from low to high. Therefore, based on the conductors’ definition due to their resistivity, they can be bifurcated into two categories.
- Low resistivity/high conductivity materials
- High resistivity/low conductivity materials
Based on Nature of Material
According to the definition of conductors, whether the material of the conductor is solid or liquid in nature, conductors can be divided into two categories.
- Solid Conductors – Examples are: Gold, Aluminium, Graphite, etc.
- Liquid Conductors – Examples are: Saline water, Mercury, etc.
Facts about Conductors
Did you know Graphite is the only non-metal that can conduct electricity? Ever wondered why?
Because in the atomic structure of graphite, out of four carbon atoms, there is always one free electron available to conduct electricity.
Therefore, as a result, it can easily conduct electricity while other non-metals cannot.
Based on Composition of Material
Before making the final composition of conductors, there are various important factors that are strictly taken into consideration such as flexibility, tensile strength, mechanical strength, production cost, etc.
Therefore, depending on the need of the environment, the composition of electrical conductors is chosen accordingly.
- Phosphor Bronze Conductors
- Cadmium Copper Conductors, etc.
Examples of Conductors
If you think you can’t relate to the real-life electrical conductors’ examples, well, here is you chance to think again!
- Aluminum
- Steel
- Mercury
- Brass
- Graphite
- Gold
- Copper
- Bronze
- Iron
- Platinum, etc.
Check Out: Zener Diode Introduction – a Brief Review
Applications of Conductors
Conductors are quite useful in our day-to-day life. In other words, you can see so many real-life applications of conductors around you. Here is a list of conductors which are the most used ones.
- Copper is commonly used for making electrical appliances such as motor winding, cables, etc.
- Mercury is used as a conducting material in a thermometer.
- Aluminum wires for power transmission and distribution.
- Silver is used for making satellites.
- Aluminum foils for food storage, etc.
Facts about Conductors
Did you know that according to the definition of conductors, when we apply a sufficiently large potential difference across the insulator, an insulator can actually behave like an electrical conductor? WHY??
Because when we apply sufficiently large voltage, the applied electric field can tear away the electrons from the atoms of an insulator.
Hence, an insulator becomes a conductor. This property of an insulator is commonly known as the breakdown voltage of an insulator.
That’s it for this post. If you like this article, share it if you like, like it if you share it. You can also find us on Mix, Twitter, Pinterest, and Facebook. Hey man, If you have come this far, do give us feedback in the comment section. It would make my day. You can also make a donation. Your donations will help us to run our website and serve you BETTER. Cheers!!!
