Gay Lussac Law (Constant Volume) is one of the three special cases of Ideal Gas Law. The other two are Boyle’s Law (Constant Temperature) and Charles Law (Constant Pressure).
When Gay Lussac Gas Law is added with Charles Law and Boyle’s Law, develops into Combined Gas Law. On the other hand, when all three special cases of Ideal Gas law is further combined with Avogadro’s Law yields Ideal Gas Law.
What Is Gay Lussac Law?
Gay Lussac Law of thermodynamics is an Ideal Gas Law that basically defines how the pressure of a fixed mass of a gas is directly proportional to the absolute temperature when mass and volume are held constant.
It is also known as Pressure Law. In fact, just because this gas law defines the relationship between temperature and pressure. That’s why this gas law is also known as pressure-temperature law.
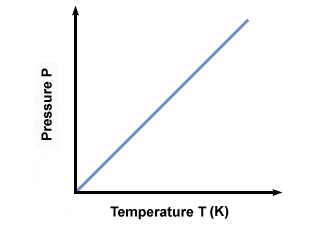
In other words, “when the volume of the sample gas is held constant, the temperature and pressure will be directly related. Meaning, the pressure of a gas will increase when heated“.
Although, like all the other Ideal Gas Laws, this pressure Law describes the behavior of an Ideal Gas. Yet, it can also be applied to real gases at normal temperatures and low pressure.
Must read, Top 6 Real-Life Applications of Boyle’s Law
This pressure and temperature relationship is known as Gay Lussac’s law; named after French chemist and physicist Joseph Louis Gay-Lussac who first proposed this law in 1808. Gay Lussac’s Law can be deduced from the Kinetic Molecular Theory Of Gas.
Real Life Gay Lussac Law Examples
If you think, you can’t relate your day to day life situations with Gay Lussac Law Example. Well, here is your chance to think again!! In fact, there are many Application Of Gay Lussac Law in everyday life, which are easy to understand. Or even you can do experiments related to them.
Firing A Bullet

In today’s world, everyone is quite aware of what is a bullet? And why they are used…!!! The working procedure of firing a bullet is based on the pressure-temperature relationship. Plus, Newton’s third law also plays its role.
Check out, Top 6 Exclusive Real-Life Examples of Sublimation
According to Gay Lussac’s law definition, if the temperature is increased, so does the pressure will. Therefore, when gunpowder burns, the ignition of gunpowder generates a large amount of superheated gas which in turn increases the pressure. As a result, the bullet travels a longer distance with high speed.
Bursting Of Automobile Tire

If you are having a car or motorbike, you will be quite aware of the heating of a tire when you are going on a long-distance journey. During hot summer days, the pressure of the well-inflated tire is almost constant.
Take a look at Top 6 Applications of Charles Law
But, when the temperature increases; the heat from the burning rubber will cause the air pressure in the tire to increase. As a result, this increase in pressure will weaken the tire wall and sometimes tire may burst.
Mathematical Representation Of Gay Lussac Law
Mathematically, Gay Lussac Law Formula is expressed as:
or
where,
P = Pressure of the gas
T = Temperature of gas (in Kelvin)
k = Constant
If we have to compare the same substance under two different conditions, then Gay Lussac’s Law Formula can be stated as:
where,
P1 = initial Pressure
T1= initial absolute temperature
P2 = final Pressure
T2 = final absolute temperature
The above pressure temperature formula shows that an increase in temperature will be an increase in pressure in proportion; and vice-versa
Gay Lussac’s Law Example Problem
If 10.0 L of oxygen exerts 87.0 kPa at 27°C, what temperature is needed to change its pressure to standard pressure?
ANS = as we know standard pressure = 101.325 kPa. Now, according to the Gay Lussac’s Law Definition:
where,
P1 = 87.0 kPa
P2 = 101.325 kPa
T1 = 300K (27 + 273)
T2 =?
now putting all the values in the above Gay Lussac’s Law Formula we get,
87 x T2 = 101.325 x 300
on solving,
Temperature T2 = 349.39 or 340 Kelvin
Editor’s Choice: Dalton’s Law – The Law of Partial Pressure
That’s it for this post. If you like this article, share it if you like it, like it if you share it. You can also find us on Mix, Twitter, Pinterest, and Facebook.
You might also like:
- Sublimation Definition, Process, Facts & Examples
- Evaporation vs Condensation (Tabular Form)
- Top 6 Verified Examples of Evaporation in Daily Life


