Evaporation is a type of phase transition in which a substance directly changes from a liquid state to its gaseous state. Unlike boiling, Evaporation is a surface phenomenon. In other words, the process of evaporation can occur at any temperature.
In fact, the higher will be the temperature, the higher will be the rate of evaporation. The opposite of evaporation is known as condensation. When a substance or material directly changes from a gaseous state to its liquid state is known as condensation.
Moreover, the basic difference between evaporation and condensation is that evaporation is a cooling process and condensation is a warming one. In addition, evaporation is an endothermic process and condensation is an exothermic one.
Editor’s Choice: Endothermic vs Exothermic – What’s the Difference?
Real Life Examples of Evaporation – Top 6
- Evaporative Coolers
- Drying of Wet clothes
- Working of a Pressure Cooker
- Formation of Salt
- Melting of Ice Cubes
- Formation of the Water Cycle
Evaporative Coolers

Evaporative coolers are nothing but a device that helps to cool down the temperature of the air, of course, with the help of the process of evaporation of water. The physics of evaporative coolers is quite simple.
According to the evaporation definition, water will have to absorb an ample amount of heat to change its liquid phase to the gaseous one. And for that, the heat is absorbed from the air that passes through the cooler.
Check out the Top 6 Most Common Examples of Condensation
In other words, when the fan & pump of the air cooler is run simultaneously, the water absorbs the heat from the air. Therefore, as a result, the temperature of the dry air drops significantly, giving a cool breeze of air.
Drying of Wet Clothes

Well, have you ever wondered why wet clothes become dry when put under direct sunlight? Yup, you are right. It’s because of the cooling effect of evaporation. When wet clothes are put under direct sunlight, the water droplets inside the clothes get heated up.
Therefore, as a result, a wet cloth becomes dry. In other words, when the hot air (due to sunlight) is blown through the wet clothes, water evaporates very rapidly.
Working of a Pressure Cooker

Yup, you heard me right. I’m not lying. Without the process of evaporation, the pressure cooker won’t make the perfect dish for us. CONFUSED?? Well, don’t be!!
When we apply heat, as a result, the water inside the pressure cooker vapourises. Hence, steam comes out of it due to evaporation.
One more thing, don’t forget to seal off the pressure cooker before the application of heat. Otherwise, there won’t be enough pressure generated inside the pressure cooker to speed up the process of cooking.
Not to mention, the working of a pressure cooker also comes in my list of Top 6 Real-Life Gay Lussac Law Examples. WHY? Because not only depends upon the process of evaporation. On the other hand, it also depends on the Gay Lussac Law of Thermodynamics.
Formation of Salt

No one can deny the fact that salt is essential for life in general. I mean, without the use of salt, our food will become tasteless. Salt can be easily prepared by simply evaporating the seawater. Salt can be prepared by using either a natural or an industrial process, of course, with the application of evaporation.
In other words, salt crystals can be easily mined from the vast sedimentary deposits. A deposit that had been laid down over the millennia from the evaporation of lake or seawater. Or else, it can be directly prepared by simply evaporating the seawater.
Must read, Top 6 Exclusive Sublimation Examples in Daily Life
Melting of Ice Cubes
 The melting of ice cubes is another real world example of evaporation. When you take an ice cube out of your refrigerator, you see that after a minute or so, it starts to change its shape from a solid to a liquid. Well, have you ever wondered why it happens?
The melting of ice cubes is another real world example of evaporation. When you take an ice cube out of your refrigerator, you see that after a minute or so, it starts to change its shape from a solid to a liquid. Well, have you ever wondered why it happens?
It happens because of the process of evaporation. As the temperature of the surroundings of an ice cube increases, an ice cube i.e a solid transforms itself into water i.e a liquid.
In fact, if the temperature of its surroundings increases to its threshold, an ice cube will completely evaporate. Therefore, will vanish into the thin air.
Formation of the Water Cycle
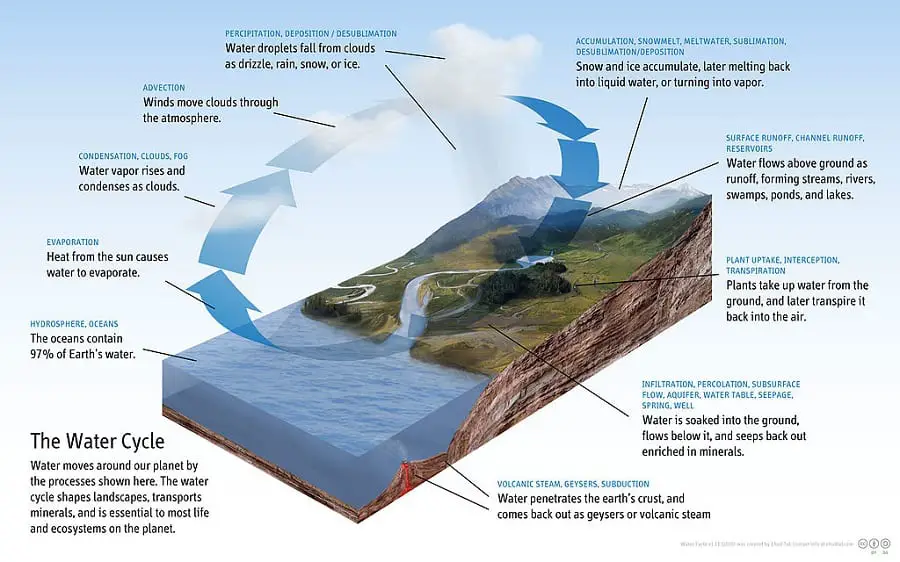 The water cycle is the most important one for the survival of life on planet earth. Why? Because evaporation is (in parts) responsible for the formation of clouds, hence, completes the water cycle. In other words, without the process of evaporation, the water cycle will cease to exist. Well, I’m not saying that only evaporation is responsible for the formation of clouds and the water cycle.
The water cycle is the most important one for the survival of life on planet earth. Why? Because evaporation is (in parts) responsible for the formation of clouds, hence, completes the water cycle. In other words, without the process of evaporation, the water cycle will cease to exist. Well, I’m not saying that only evaporation is responsible for the formation of clouds and the water cycle.
There are others too that are responsible for the steady completion of the hydrological cycle. The other processes are condensation, sublimation, precipitation, transportation, etc. See the above image for a proper understanding.
A water cycle is nothing but the continuous movement of water from within, outside, and, on the surface of the earth. The Sun heats water in the seas and oceans. As a result, water from the ocean and seas evaporates to form water vapor in the air.
When the air is cooled to its dew point and becomes saturated. Water vapor in the air condenses to form clouds. Later, these clouds precipitate back to earth in the form of rain, snow, or ice pellets.
Some Other Real World Examples of Evaporation
- Evaporation of sweat from our body
- The smoke coming out of a cup of hot coffee
- Food preservation
- Evaporation of a nail paint remover
- Evaporation rate of ethanol
- Drying a wet floor
- The process of distillation
- Drying of river beds
- Hairdryers
- Ironing of clothes, etc.
Frequently Asked Questions
1. What is Evaporation?
Ans. Evaporation is a type of phase transition in which a substance directly changes from a liquid state to its gaseous state.
2. What are the factors that affect evaporation?
Ans. Apart from temperature, the factors that affect the rate of evaporation are as follows
- humidity
- surface area
- pressure
- the concentration of the substance
- air movement, etc.
3. How to make water evaporate faster?
Ans. In order to increase the evaporation rate of water, before applying heat, it is advised to spread the water over a large surface area.
Moreover, the humidity should also be controlled. In other words, the more humidity, the less will be the evaporation rate of water.
4. How does evaporation cause cooling?
Ans. The process of evaporation causes cooling naturally. According to the definition of evaporation, in order to change its phase from liquid to gaseous state, a liquid (say water) has to absorb an ample amount of heat (energy) from its surrounding.
Therefore, as a result, bringing down the temperature of the surrounding. Hence, causing the cooling effect.
That’s it for this post. If you like this article, share it if you like, like it if you share it. You can also find us on Mix, Twitter, Pinterest, and Facebook.