Anything that has mass and occupies space is known as matter. Based on properties like whether material is good or a bad conductor of heat and electricity, a matter or simply an element can be bifurcated into two groups. These are known as Metals and Nonmetals. So, what is the difference between metals and nonmetals?
The primary difference between metals and nonmetals is that metals are good conductors of heat and electricity. Nonmetals, on the other hand, are bad conductors of heat and electricity, hence, insulators. The other significant difference between the two is that metals are opaque and nonmetals are transparent.
No wonder, there are so many differences as well as similarities between them. There are some exceptions between them such as there are some metals that are bad conductors of heat and electricity. Similarly, some nonmetals are good conductors of heat and electricity.
Today, in this exclusive article, I am gonna walk you through all the details regarding both metals and nonmetals. But before going ahead, let me give you a brief review of the two in a tabular form. Let’s dive right in…!!!
Metals vs Non-metals
| Metals | Non-metals | |
| 1. | Metals are good conductors of heat and electricity except for Tungsten. | Non-metals are bad conductors of heat and electricity except for Graphite. |
| 2. | They are shiny except for Potassium. | They are dull except for Iodine. |
| 3. | They are sonorous. | They are non-sonorous. |
| 4. | Metals are hard except for Sodium. | Non-metals are soft except for Diamond. |
| 5. | Metals are electropositive. | Nonmetals are electronegative. |
| 6. | They are ductile and malleable. | They are non-ductile and brittle. |
| 7. | Metals have high density and high melting points. | Nonmetals have low density and low melting points. |
| 8. | Metals are solid at room temperature, except for Mercury. | Non-Metals can be either solid, liquid, or, gas at room temperature. |
| 9. | They can be corroded easily. | They cannot be corroded easily. |
| 10. | Examples of metals are Iron, Copper, Silver, Gold, Platinum, Zinc, Nickel, Tin, etc. | Examples of nonmetals are Hydrogen, Helium, Oxygen, Sulphur, Chlorine, Argon, Radon, etc. |
What are Metals?
By definition, metals are opaque and shiny elements (materials) that are normally good conductors of heat and electricity. Of course, there are some exceptions too. Such as, in terms of conductivity, Tungsten is a poor or bad conductor of heat and electricity.
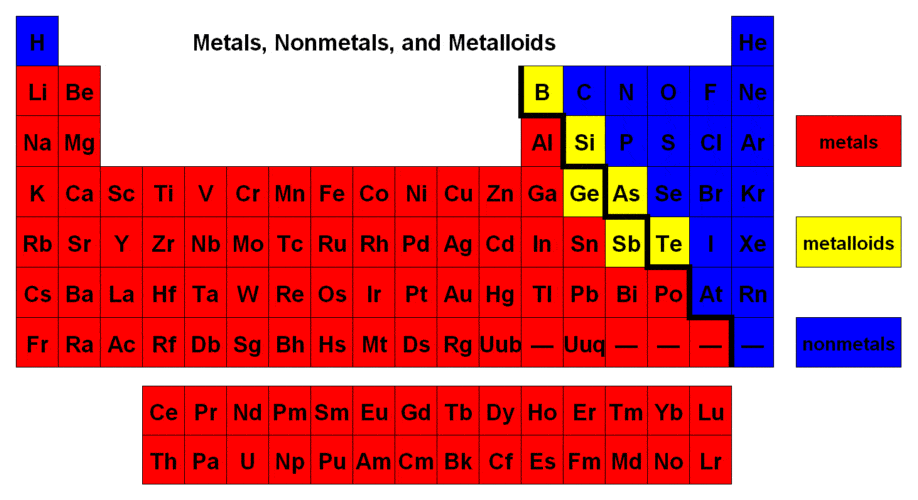
Similarly, in terms of lustrousness, potassium is the only metal that does not have a shining surface. From the above diagram, you can easily see which one of the periodic table elements is metals. Such that, all the elements that are shown in red color are metallic.
Why metals are electropositive?
Metals are electropositive, WHY? Because to form positively charged ions, they tend to lose electrons. One can also say that metals are electropositive because they can lose their valence electrons from their outermost shell. Out of all, Caseum is the most electropositive metal available on Earth.
Physical Properties of Metals
Some of the physical properties of metals are as follows:
- Metals are good conductors of heat and electricity
- Shiny
- Opaque
- Ductile
- Sonorous
- Metals are solid at room temperature, except for Mercury
- High density
- High melting point
- Malleable, etc.
Chemical Properties of Metals
Some of the chemical properties of metals are as follows:
- Metals form basic oxides
- Can be easily corroded
- Metals are good for reducing agents
- Metals are highly electropositive
- They react with water easily, etc.
Editor’s Choice: Difference Between Ferrous and Non-Ferrous Metals in Tabular Form
What are Non-Metals?
By definition, non-metals are transparent and non-lustrous (dull-looking) elements or materials that are normally bad conductors of heat and electricity. That’s why non-metals are also known for making insulators. Just like metals, non-metals also have some exceptions too.

Such that, in terms of conductivity, Graphite is the only non-metal that can conduct heat and electricity. Similarly, in terms of lustrousness, Iodine is the only non-metal that has a shiny surface. Hence, can be easily polished.
From the above diagram, you can easily see which one of the periodic table elements is non-metals. Such that, all the elements that are shown in blue color are non-metallic.
Why nonmetals are electronegative?
Non-metals are electronegative, WHY? Because to form negatively charged irons, they tend to gain electrons. One can also say that non-metals are electronegative because they can gain electrons in their outermost shell. Out of all, Fluorine is the most electronegative non-metal available on earth.
Physical Properties of Non-Metals
Some of the physical properties of non-metals are as follows:
- Non-Metals are bad conductors of heat and electricity
- Dull-looking
- Non-Ductile
- Transparent
- Non-Sonorous
- Non-Metals can be solid, liquid, or, gas at room temperature
- Low density
- Low melting point
- Brittle, etc.
Chemical Properties of Non-Metals
Some of the chemical properties of nonmetals are as follows:
- Non-Metals form acidic oxides
- Cannot be corroded easily
- Non-Metals are highly electronegative
- They do react with water easily
- Non-metals are good oxidizing agents, etc.
That’s it for this post. If you like this article, share it if you like, and like it if you share it. You can also find us on Mix, Twitter, Pinterest, and Facebook. Hey man, If you have come this far, do give us feedback in the comment section. It would make my day. You can also make a donation. Your donations will help us to run our website and serve you BETTER. Cheers!!!
