Charles Law Of Thermodynamics (Constant Pressure) is one of the three special cases of Ideal Gas Law. The other two gas laws are Boyle’s Law (Constant Temperature) and Gay-Lussac’s Law (Constant Volume).
When Charles Law is substituted with Boyle’s Law and Gay-Lussac’s Law, develops into Combined Gas Law; further combined with Avogadro’s Law yields Ideal Gas Law.
What Is Charles Law Of Thermodynamics?
Charles Law of thermodynamics is an Ideal Gas Law that basically defines how the volume of a fixed mass of a gas is directly proportional to the temperature when pressure held constant.
That’s why this relationship between volume and temperature is also known as the Law Of Volumes.
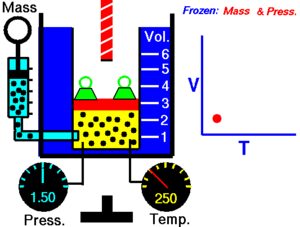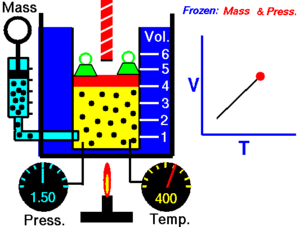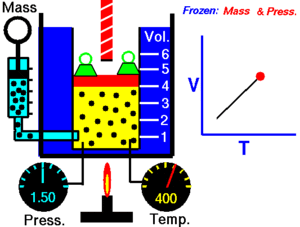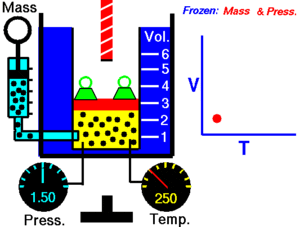
Although, like all the other Ideal Gas Law, Charles gas law describes the behavior of an Ideal Gas. Yet, it can also be applied to real gases at normal temperatures and low pressure.
Take a look at Top 6 Exclusive Real-Life Examples of Sublimation
In other words, according to Charle’s law, the volume of a gas is directly proportional to temperature at constant pressure.
This phenomenon is known as Charles’s law of thermodynamics; named after French Scientist, Inventor, and Mathematician Jacques Charles who first proposed this law in his unpublished work in the 1780s.
In fact, Jaques Charles Law can be deduced from the Kinetic Molecular Theory Of Gas.
Real-Life Examples Of Charles Law
Well, if you think, you can’t relate to the real-life examples of Charles law, here is your chance to think again!! In fact, there are several Charles Law real-life applications in everyday life, which are easy to understand or even you can do experiments related to them.
Frankly speaking, I can’t explain all of them in this article. That’s why I have written an exclusive article based on Examples of Charles law in real life. I know you will love it.
Pop Up Turkey Thermometer

Well, the working of Turkey Pop Up Timer is based on Charles Law Of Thermodynamics. According to Charles’s Law definition, gas tends to expand when heated”, the same phenomenon works for Pop Up Turkey Thermometer.
Related, Top 6 Examples Of Boyle’s Law In Everyday Life
The turkey thermometer is placed inside the turkey. As the turkey cooks, the gas inside the thermometer expands with an increase in temperature. Therefore, the turkey thermometer pop up; indicating that the turkey is cooked and ready to serve.
Helium Balloon On Chilly Day

Obviously, you are quite aware of what is helium balloon? In fact, we all remember that during our childhood days when we step outside our home with a helium balloon on the chilly days (winter season of course).
Related, Top 6 Real-Life Gay Lussac law Examples
The balloon will shrink a bit due to the degree of coldness or decrease in temperature. It happens because of Charles Law. As Charles’s law states that when the temperature decreases so do the volume of helium gas inside a balloon.
On the other hand, when the same balloon is brought back to a worm room, it regains its original shape.
Charles Law Formula
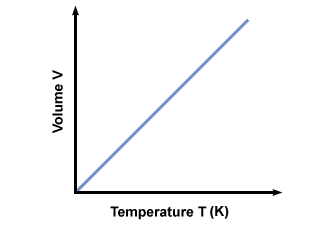
Mathematically, Charles Law formula is expressed as
or
where,
V = volume of the gas
T = temperature of the gas (in kelvins)
k = Charles law constant
If we have to compare the same substance under two different conditions, then Charles Law equation can be stated as
where,
V1 = initial volume
T1= initial absolute temperature
V2 = final volume
T2 = final absolute temperature
The above equation shows that “an increase in temperature will be an increase in volume in proportion; and vice-versa”.
Charles Law Example Problem
A gas occupies 300 cm3 at a temperature of 0 C and pressure of 670 mm Hg. What will its volume be at 100 C?
ANS = as we know
where,
V1 = 300 cm3
T1 = 273K (0 + 273)
T2 = 373K (100 + 273)
now putting all the values in the above Charles law formula we get,
300 x 373 = V2 x 273
on solving,
final volume V2 = 409.89 cm3
Editor’s Choice: Dalton’s Law – The Law of Partial Pressure
That’s it for this post. If you like this article, share it if you like it, like it if you share it. You can also find us on Mix, Twitter, Pinterest, and Facebook.



