What is Zeroth’s Law of Thermodynamics?
The Zeroth Law Of Thermodynamics is one of the most important physical laws of thermodynamics which states that if two thermodynamic systems are in thermal equilibrium with the third thermodynamic system, then they all are in thermal equilibrium with each other.
Must read, Top 6 Applications Of Charles Law
In other words, if system A is in thermal equilibrium with system B. On the other hand, system B is in thermal equilibrium with system C. Then according to Zeroth law of thermodynamics definition, system A is Bound to be in thermal equilibrium with system C.
What is Thermal Equilibrium?
According to the thermal equilibrium definition, when two thermodynamic systems or simply speaking two thermal bodies are in thermal contact with one another, separated by a barrier (permeable only to heat). Then, there will be no transfer of heat energy between two physical systems.
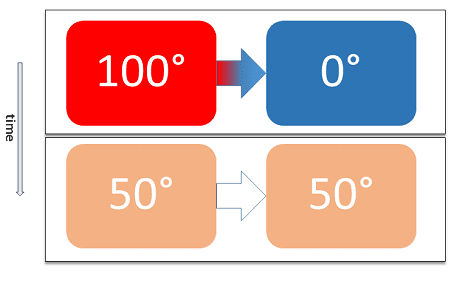
Don’t get confused (see the above image). I mean, practically there will be a transfer of heat from one system to another. But, the net transfer of heat will be equal to zero.
History of Zeroth Law of Thermodynamics
Years before the discovery of the Zeroth Law, the whole scientific community was well aware of the other Laws of thermodynamics. The 3 laws of thermodynamics are – First Law of thermodynamics, the Second Law, and, Third Law Of Thermodynamics.
Why Is The Zeroth Law Of Thermodynamics Important?
As I said, the whole scientific community was well aware of the theory of thermodynamics. But none of the available theories was able to accurately define the temperature. Therefore, when Zeroth Law was discovered, it superseded all the other laws of thermodynamics to accurately define the temperature.
Recommended, Top 6 Naming Of Chemical Elements After Famous Scientists
For this reason, the definition of temperature given by the Zeroth law is regarded as the empirical definition of temperature.
Not to mention, the old laws of thermodynamics were even given their respective nomenclature (like 1st, 2nd, 3rd). With this in mind, the whole scientific community was puzzled by the fact that the newly discovered law is far superior to the original 3 laws of thermodynamics.
In that case, zeroth law should be named above all in the existing literature. But it would have created a mess. Simply because the other 3 laws of thermodynamics were well known by their assigned nomenclature. Therefore, renumbering them would obviously create a conflict among the whole scientific world.

Finally, in the year 1935, this mess was ended by the British physicist and astronomer Ralph H. Fowler who formulated the name known as Zeroth Law.
Zeroth Law of Thermodynamics Examples
Well, in my view, in order to find something; we don’t need to think deeply. All we need to do is think clearly. The same goes for Examples Of Zeroth Law Of Thermodynamics.
I mean, there is so many zeroth law of thermodynamics examples that can be seen in our everyday life. And the most common example is Thermometer.
Working of Thermometer

I hope you are well aware of the fact that what is a thermometer. And how a thermometer works? But the thing you don’t know about thermometers is their working principle. The working of the thermometer is solely based on the zeroth law.
A thermometer is the perfect example of the Zeroth Law Of Thermodynamics. If you don’t believe me check yourself. Just put a neutral thermometer under your tongue for say one minute. You will see that whatever your body temperature is, is will be shown on the thermometer.
Highly Recommended, Top 6 Real-Life Gay Lussac Law Examples
The other day-to-day example will be – like take a glass of normal water (say at 27 degrees Celsius) and boiling water (say at 100 degrees Celsius) and putting them in your freeze for 4 or 5 hours.
Later you will see that the normal water and the boiling water have reached thermal equilibrium with respect to the temperature of the freeze.
That’s it for this post. If you like this article, share it if you like it, like it if you share it. You can also find us on Mix, Twitter, Pinterest, and Facebook.
You might also like:
- Charles Law of Thermodynamics – The Law of Constant Pressure
- Boyle’s Law of Thermodynamics – The Law of Constant Temperature
- Gay Lussac Law of Thermodynamics – The Law of Constant Volume