Wanna know what are some exclusive examples of exothermic reactions in everyday life? If yes, then you are at the right place at the very right time. But, before going ahead, let me give you a short and crisp review about the fact that what is exothermic reaction anyway!!!
An exothermic reaction is a type of chemical reaction during which a system releases energy to its surroundings. One can also say that in the case of an exothermic process, the change in enthalpy is always negative. The opposite of an exothermic reaction is an endothermic reaction.
An endothermic reaction is a type of chemical reaction during which a system absorbs energy from its surroundings. One can also say that in the case of an endothermic process, the change in enthalpy is always positive.
Editor’s Choice: Difference Between Endothermic and Exothermic Reactions
Examples of Exothermic Reactions in Everyday Life
If you think, you can’t relate to examples of exothermic reactions in everyday life. Well, here is your chance to think again…!!!
- Rusting of Iron
- Combustion
- Condensation
- Nuclear Reactions
- Deposition
- Instant Hot Pack
Rusting of Iron

Rusting of iron typically occurs due to the chemical reaction between any form of iron or its alloy with moist air. During the process of rusting, iron chemically combines with the oxygen present in the most air-generating heat. Hence comes under the category of exothermic reactions.
No wonder just because the whole process usually occurs at a very slow pace, it is quite hard to detect the release of heat into the atmosphere. Not to mention, any type of redox reaction or simply rusting of any metal is an exothermic reaction.
Combustion

Any type of combustion is an exothermic reaction. Whether you are burning wood, coal, or even petroleum products, all of them release energy in the form of heat or light.
For example, when we burn coal in a coal power plant, the process of combustion takes place due to the presence of oxygen which in turn produces a lot of heat that helps to vaporize water to generate steam.
The steam is then released towards the blade of the turbine which by rotating itself starts off the electric generator to produce electricity. You can see that without the process of combustion which is an exothermic reaction, we won’t be having electricity in our homes.
Editor’s Choice: Coal vs Solar Power: Which is Better?
Condensation
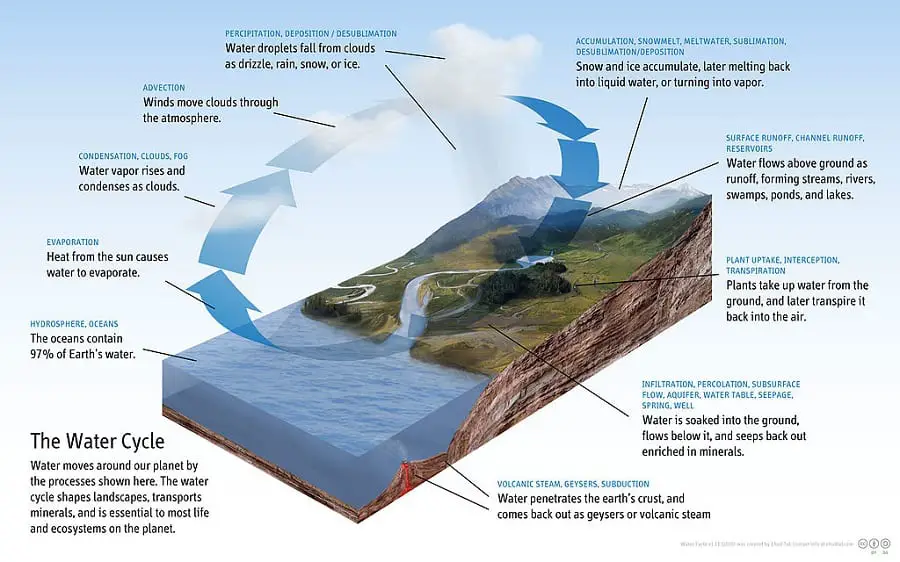
The very next one in my list of exclusive exothermic reaction examples is the process of condensation. No wonder you would be thinking how. Okay, let me explain how…!!!
A typical example of condensation would be that water vapor rises and condenses as clouds. Condensation is a type of phase transition during which a substance changes from its gaseous state to its liquid state.
So, when the water vapors (gas) condense to form clouds (liquid), the molecules of the water vapors give up their heat energy to the surroundings. In other words, to transform from a gaseous phase into a liquid phase, it must release energy in the form of heat.
Nuclear Reactions
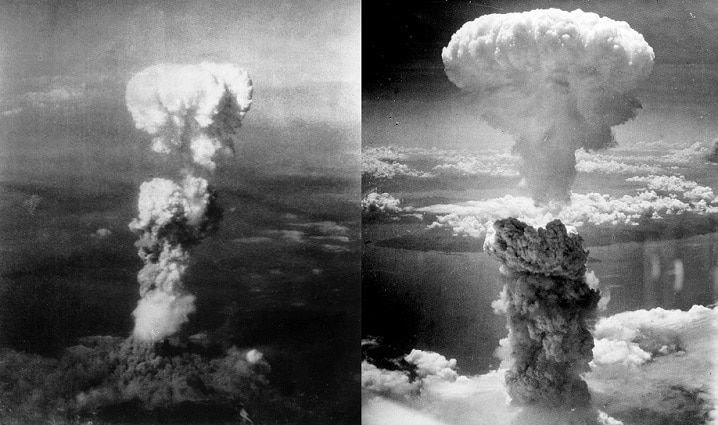
Whether it’s fission or fusion of atoms, both ways, we are creating energy. In other words, all type of nuclear reactions produces energy in the form of heat. Therefore, comes under the category of exothermic reactions. Just to let you know a nuclear fission reaction can further be divided into two subcategories.
And, so does the nuclear fusion reaction. When a large amount of energy is produced at once by splitting atoms, we call this process an uncontrolled chain reaction in nuclear fission. A typical example would be the nuclear explosion of Hiroshima and Nagasaki.
On the other hand, when the energy is slowly produced by splitting atoms in a controlled or sustained environment, we call this process a controlled chain reaction in nuclear fission. A typical example would be electricity-producing nuclear power plants.
Similarly, when a large amount of energy is produced at once by fusing atoms, we call this process an uncontrolled chain reaction in nuclear fusion. A typical example would be thermonuclear weapons or hydrogen bombs.
On the other hand, when the energy is slowly produced by fusing atoms in a controlled or sustained environment, we call this process a controlled chain reaction in nuclear fusion. To date, a reactor that can sustain a controlled nuclear fusion is still in its development phase.
Editor’s Choice: 6 Fun Facts about Nuclear Energy in SIX Minutes
Deposition

Just like condensation, the process of deposition is also an exothermic reaction example. A deposition is a type of phase transition during which a substance directly changes from a gaseous state to its solid state releasing energy in the form of heat. A typical example of deposition would be the formation of snow.
So, when the water vapor (gas) in the clouds changes directly to ice (solid), the molecule of the water vapor releases the heat energy to the surroundings. This is why the air feels warmer when it’s snowing.
Instant Hot Pack

We all have used it at least once in our life. There are times in our lives when we have to go through some harsh winter season. During winter, we ought to use hot packs. Not to mention, we can also use instant hot packs to soothe ourselves from any kind of unbearable pain.
Be it a sprain in the neck or back, you get an instant hot pack. So what is this hot pack anyway? More importantly, how does it qualify to be on the list of exothermic reaction examples?
An instant hot pack is a pack of two pouches separated by a thin membrane. The inner pouch contains water. The outer one contains ionic salt such as magnesium sulfate or calcium chloride. When the thin membrane is broken, the salt gets dissolved in water.
As a result, the ionic bonds of the salt separate resulting in heating the water. In other words, when calcium chloride or magnesium sulfate gets mixed with water, the reaction between them releases energy in the form of heat, making the whole process an exothermic reaction.
Editor’s Choice: Endothermic Reaction Examples in Everyday Life (ALL NEW)
Some other Examples of Exothermic Reaction in Everyday Life
Apart from the above-mentioned ones, I am also mentioning some of a few here.
- Respiration
- Acid Dissolving in Water
- Formation of Ice Cubes
- Dew on Grass
- Burning of Candle
- Firework
- Decomposition of Vegetables, etc.
That’s it for this post. If you like this article, share it if you like, like it if you share it. You can also find us on Mix, Twitter, Pinterest, and Facebook. Hey man, If you have come this far, do give us feedback in the comment section. It would make my day. You can also make a donation. Your donations will help us to run our website and serve you BETTER. Cheers!!!
You might also like:
- 6 Examples of Chemical Energy in Daily Life
- Examples of Molecules Made Simple: A Quick Reference Guide
