An endothermic reaction is a type of chemical reaction during which a system absorbs energy from its surroundings. One can also say that in the case of an endothermic process, the change in enthalpy is always positive.
The opposite of an endothermic reaction is an exothermic reaction. An exothermic reaction is a type of chemical reaction during which a system releases energy in its surroundings. One can also say that in the case of an exothermic process, the change in enthalpy is always negative.
Editor’s Choice: Difference Between Endothermic and Exothermic Reactions
10 Endothermic Reaction Examples in Everyday Life
If you think, you can’t relate to examples of endothermic reactions in daily life. Well, here is your chance to think again…!!!
- Evaporation
- Photosynthesis
- Fusion of Elements Heavier than Iron
- Sublimation
- Electrolysis
- Instant Cold Pack
- Melting of Ice
- Cooking Food
- Baking Soda and Vinegar Reaction
- Salt Dissolving in Water
Evaporation

Evaporation is a type of phase transition in which a substance directly changes from a liquid state to its gaseous state.
In the case of drying wet clothes, the water droplets inside the clothes (system) pull the heat from the surroundings. As a result, the enthalpy or internal energy of the system (wet clothes) increases.
Photosynthesis

Unlike animals (including us), plants make their own food. The process by which plants make their food is known as photosynthesis which is also considered the result of an endothermic reaction.
During the process of photosynthesis, solar energy or sunlight gets absorbed by the leaves which later with water and carbon dioxide is converted into food. Therefore, as a result, a plant grows.
Fusion of Heaver Elements than Iron

Yeah yeah, lighter elements like hydrogen and its isotopes are fusible only. Well, let me tell you, something brother…!!! It’s not true. All elements are capable of fusing with each other regardless of their size. But, there is a catch.
Since we humans are trying to extract energy by fusing elements in a fusion reactor, we have to use lighter elements like hydrogen and its isotopes. What I mean is that in order to extract energy in a nuclear fusion reactor, the reaction has to be exothermic.
And, for that, we have to use the lightest elements. However, as per the available scientific data, a typical case of endothermic reaction would be a nuclear fusion of elements heavier than iron in supernovae.
Sublimation

A typical example of sublimation would be the sublimation of dry ice. Just to mention, dry ice is nothing but a solid form of carbon dioxide.
Sublimation is a type of phase transition in which a substance directly changes from the solid state to the gaseous state, bypassing the liquid state. In the case of dry ice, in order to sublimate, solid carbon dioxide (dry ice) extracts the heat from the surrounding making it an endothermic process.
Electrolysis
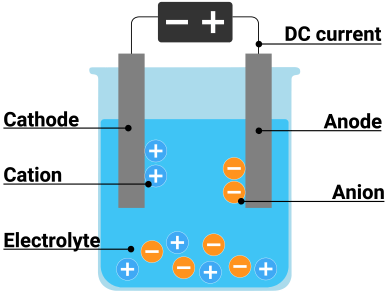
Electrolysis is a process that uses an electric current to drive an endothermic chemical reaction. It is commonly used to split water into hydrogen and oxygen gases.
The electrical energy provided to the system is absorbed by the reaction, causing a decrease in temperature.
Instant Cold Pack
Let’s talk about something painful. Have you ever felt a sprain in your neck or shoulder? Or ever had back pain? What you would need to relax instantly from that unbearable pain? Any guess? Well, You need an Instant Cold Pack…!!!
As the name suggests, an instant cold pack is basically a pack of two pouches separated by a thin membrane which we primarily use to ease the pain that we get from a sore back or sprained ankle.
One of them contains water and the other one contains Ammonium nitrate. Now you would be thinking that how an instant cold pack comes under the category of an endothermic reaction. Okay, let’s see how this works!
When the thin membrane is broken which was separating both the water and Ammonium Nitrate from mixing together, a chemical reaction takes place between them, which in turn makes the water cold as ice, hence helping us to ease our sore back or sprain in an ankle.
In other words, when Ammonium nitrate is mixed with water, the reaction between them takes away the heat from the water, making the whole process an endothermic reaction.
Melting of Ice

One of the most recognizable endothermic reactions is the melting of ice. When solid ice absorbs heat from its surroundings, it undergoes a phase change and transforms into liquid water.
This process requires energy input, which is taken from the surroundings, causing a decrease in temperature.
Cooking Food

The process of cooking food is an endothermic reaction. Suppose you are making a Maggi. During the process of cooking, the energy of the pan is utilized or I can say absorbed by the Maggi.
Hence making it an endothermic process. Speaking of Maggi, now I am feeling hungry after a long day. I better wrap this one quickly!!!
Baking Soda and Vinegar Reaction

The reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) is a well-known endothermic reaction.
When these two substances are mixed, they produce carbon dioxide gas, water, and sodium acetate. As a result, the reaction absorbs heat, causing the surroundings to cool down.
Salt Dissolving in Water

Last but not least one on my list is the dissolving of salt in water. When salt (sodium chloride) dissolves in water, it undergoes an endothermic reaction.
The dissolution process absorbs heat energy from the surroundings, resulting in a temperature decrease.
Editor’s Choice: Exothermic Reaction Examples in Everyday Life (Exclusive)
That’s it for this post. If you like this article, share it if you like, like it if you share it. You can also find us on Mix, Twitter, Pinterest, and Facebook. Hey man, If you have come this far, do give us feedback in the comment section. It would make my day. You can also make a donation. Your donations will help us to run our website and serve you BETTER. Cheers!!!
You might also like:
- 6 Examples of Chemical Energy in Daily Life
- Examples of Molecules Made Simple: A Quick Reference Guide
