In physical engineering and chemical science, there are mainly two types of chemical bonds. These are Ionic Bonds and Covalent bonds. In this exclusive article, I am only going to explain the definition of Covalent Bond.
However, I will still clear some air regarding the basic difference between covalent and ionic bonds. See, a covalent bond is sharing of electrons between two atoms. On the other hand, an ionic bond is a complete transfer of valence electrons between two atoms.
Just to let you know, apart from ionic and covalent bonds, there are two more types of chemical bonds. These are hydrogen bonds and polar bonds.
Editor’s Choice: Ionic Bond vs Covalent Bond – Difference and Comparision
What is Covalent Bond?
By definition, a Covalent bond is a type of chemical bond that occurs due to the sharing of electrons between the participating atoms.
Not to mention, these types of chemical bonds can only occur between non-metallic elements having the same or almost equal electronegativity values.
Due to the equal electronegativity value of both participating atoms, there is no transfer of electrons. Therefore, as a result, the formation of ions does not take place in covalent bonding.
Editor’s Choice: Ionic Bond Definition, Properties, Examples & Uses
Additionally, the participating pairs of electrons in covalent bonding are known as shared pairs or simply bonding pairs.
Types of Covalent Bonds
Based on the number of shared electron pairs, by definition, a covalent bond can be further bifurcated into three types. Let us get to know them in detail.
Single Covalent Bond
When there is a formation of a covalent bond due to the sharing of single pair of electrons (i.e two electrons) between participating atoms, we call it a single covalent bond.
Check Out: Amorphous Solid – Definition, Properties & Examples
They are weaker and have a low density as compared to double and triple covalent bonds. Not to mention, they are more stable than the other types of covalent bonds. It is represented by a single dash (-).
Example of Single Covalent Bond
A typical example of a single covalent bond is the hydrochloric acid molecule. A molecule of hydrochloric acid (HCL) consists of one hydrogen and one chlorine atom.
Hydrogen (H) has one valence electron in its outermost shell. Therefore, it requires one more electron to fill up its orbital. On the other hand, Chlorine (CL) has seven valence electrons in its outermost shell. Therefore, it also requires one more to fill up its orbital.
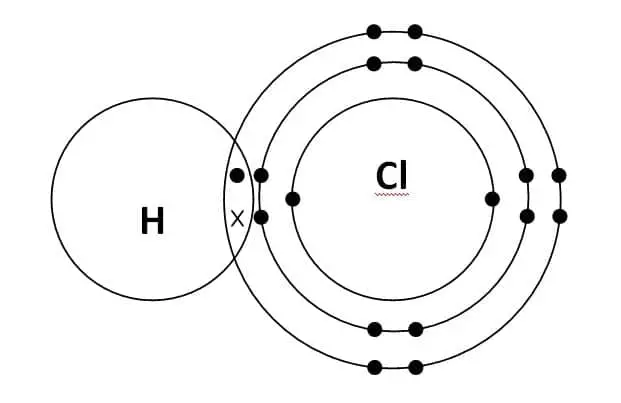
When one atom of hydrogen and one atom of chlorine combines to form a chemical bond, in order to fill up the Octet of chlorine, hydrogen shares its lone electron with the chlorine atom.
Similarly, in order to fill up the Octet of hydrogen, chlorine shares one electron with a hydrogen atom. Hence, forming a single covalent bond i.e sharing of two electrons.
Editor’s Choice: Anion vs Cation – What’s the Difference??
Double Covalent Bond
When there is a formation of a covalent bond due to the sharing of double pair of electrons (i.e four electrons) between participating atoms, we call it a double covalent bond.
They are stronger but less stable than a single covalent bond. It is represented by a double dash (=).
Example of Double Covalent Bond
A typical example of a double covalent bond is the Carbon dioxide molecule. A molecule of carbon dioxide (CO2) consists one one carbon atom and two oxygen atoms.
Carbon (C) has 4 valence electrons in its outermost shell. Therefore, it requires 4 more to fill up its orbital. On the other hand, both of the oxygen (O) atoms have six valence electrons in their outermost shell. Therefore, they both require two-two more to fill up its orbital.

When one atom of carbon and two atoms of oxygen combine to form a chemical bond, in order to fill up the Octet of carbon, both of the oxygen atoms will share two electrons each with the carbon atom.
Similarly, in order to fill up the Octet of both of the oxygen atoms, carbon will share two electrons each with the oxygen atoms. Hence, forming a double covalent bond i.e sharing of four electrons.
Triple Covalent Bond
When there is a formation of a covalent bond due to the sharing of triple pair of atoms (i.e six electrons) between participating atoms, we call it a triple covalent atom.
Must Read: Crystalline Solid – Definition, Types, Properties & Examples
They are the strongest but the least stable as compared to single and double covalent bonds. It is represented by a triple dash (≡).
Example of Triple Covalent Bond
A typical example of a triple covalent bond is the Cyanide molecule. A molecule of Cyanide is an anion (CN–) that consists of one carbon atom and one nitrogen atom.
Carbon (C) has four 4 valence electrons in its outermost shell. Therefore, it requires four more to fill up its orbital.
On the other hand, nitrogen (N) has five valence electrons in its outermost shell. Therefore, it requires three more to fill up its orbital.
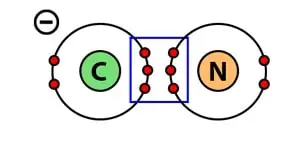
When one atom of carbon and one atom of nitrogen combine to form a chemical bond, carbon and nitrogen will share three electrons each.
Hence, forming a triple covalent bond i.e sharing of six electrons. Not to mention, an extra available electron on carbon makes Cyanide an anion.
Must Read: Difference Between Atom and Molecule in Tabular Form
Properties of Covalent Bond
There are so many properties of covalent bonds. Some of them are listed below:
- By definition, Covalent bond is directional in nature.
- They have low melting and boiling points.
- They show poor electrical and thermal conductivity.
- Covalent compounds are insoluble in water.
- Bonds are strong, therefore, a large amount of energy is needed to break them.
- Covalent bonds are formed due to sharing of electrons.
- Covalent compounds usually have a low enthalpy of vaporization or fusion, etc.
Examples of Covalent Bond
If you think you can’t relate to covalent bond examples in everyday life. Well, here is your chance to think again!
- Nitrogen (N2)
- Ammonia (NH3)
- Cyanide (CN–)
- Hydrogen (H2)
- Ethene (C2H4)
- Hydrochloric Acid (HCL)
- Ethane (C2H6)
- Ethyne (C2H2)
- Carbon dioxide (CO2)
- Oxygen (O2), etc.
Check Out: Difference Between Crystalline and Amorphous Solid
That’s it for this post. If you like this article, share it if you like, like it if you share it. You can also find us on Mix, Twitter, Pinterest, and Facebook. Hey man, If you have come this far, do give us feedback in the comment section. It would make my day. You can also make a donation. Your donations will help us to run our website and serve you BETTER. Cheers!!!
