Whatever is around you is made up of matter. In general, there are three types of matter. These are solid, liquid, and gas. A solid can be further classified into three different groups. These are Crystalline Solids, Polycrystalline Solids, and Amorphous Solids. In this exclusive article, I am going to explain the definition of Crystalline Solid.
When molecules, atoms, or subatomic particles are arranged in a highly ordered manner, we call this type of solid a Crystalline Solid. In other words, Crystalline solids have definite and regular geometrical shapes.
Editor’s Choice: Difference Between Crystalline and Amorphous Solid
What is Crystalline Solid?
According to the definition of crystalline solid, a material whose molecules, atoms, or even sub-atomic particles are arranged in a highly ordered structure is known as crystalline solids.
In other words, you can simply say that crystalline solid consists of particles that are arranged in a 3-dimensional manner. That’s why we also call them highly-ordered solids or simply true solids.
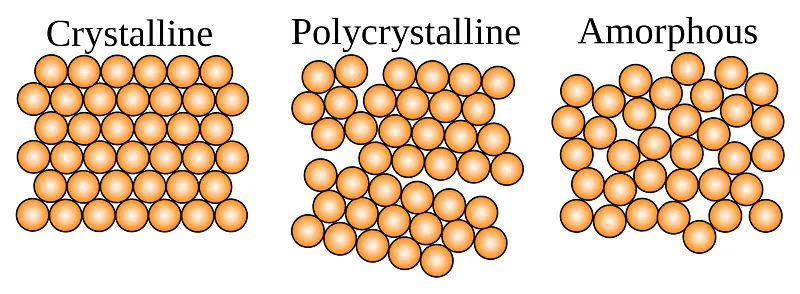
Additionally, the word crystalline solids or simply crystals is derived from the ancient Greek word “Krustallos” which simply means rock crystals. As a matter of fact, apart from being a highly ordered structure, the intermolecular forces between the constituent particles are always equal.
Check Out: Amorphous Solid – Definition, Properties & Examples
Types of Crystalline Solids
Based on the molecular forces and chemical bonding between the constituent particles, crystalline solids can be further bifurcated into four parts. Let us get to know them in detail.
Ionic Crystals
As the name suggests, the Ionic crystals consist of alternating positive and negative ions. Additionally, these types of crystalline solids are generally hard, brittle, and tend to show a very high melting point. For example, Calcium fluoride (CaF2) has a melting point of 1418 °C.

Not to mention, Ionic solids do not conduct electricity. WHY? Because in its solid-state, they do not have free electrons or ions to conduct electricity. However, in their molten state, they do conduct electricity.
Related: What are Conductors? – Definition, Types & Examples
WHY? Because their positively charged cations and negatively charged anions are free to move. Hence, can conduct electricity. Furthermore, the atoms of Ionic crystals are held together by electrostatic attraction. Some of the examples of ionic crystals are:
- Sodium Chloride (NaCl)
- Calcium fluoride (CaF2)
- Lithium bromide (LiBr)
- Caesium iodide (CsI), etc.
Metallic Crystals
According to the crystalline solid definition, metallic crystals consist of atomic nuclei surrounded by the “sea” of delocalized electrons. These delocalized electrons are nothing but valance electrons surrounding the atomic nuclei of the metallic crystals.
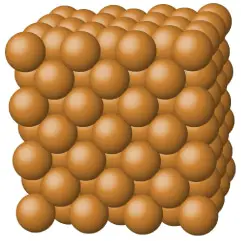
Just because these valence electrons are free to move anywhere within the given material, metallic solids are good conductors of electricity. Equally important, these types of crystalline solids have high thermal and electrical conductivity.
Must Read: Difference Between Conductors and Insulators in Tabular Form
Additionally, they also exhibit metallic luster and high malleability. The atoms of metallic crystals are held together by metallic bonds. Some of the examples of Metallic crystals are:
- Mercury (Hg)
- Copper (Cu)
- Gold (Au)
- Silver (Ag), etc.
Molecular Crystals
As the name suggests, Molecular crystals consist of a number of different molecules. Not to mention, Molecular crystals are held together by intermolecular forces between the constituent molecules.
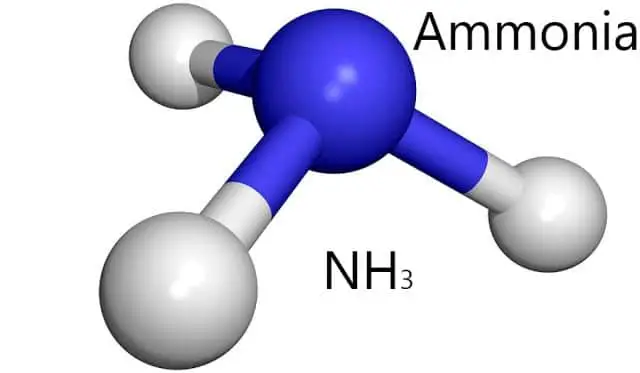
In fact, just because molecular solids form due to a number of different molecules, as a result, these types of crystalline solids have variable hardness, variable brittleness, as well as variable melting points. Some of the examples of molecular crystals are:
- Hydrogen (H2)
- Water (H2O)
- Ammonia (NH3)
- Iodine (I2), etc.
Check Out: Covalent Bond – Definition, Types, Properties & Examples
Covalent Network Crystals
According to the definition of crystalline solid, Covalent network crystals consists of atoms being covalently bonded with each other. These types of crystalline solids are not good conductors of electricity. WHY? Because there are no free electrons or ions available to conduct electricity.

In fact, just because the atoms of covalent network solids are covalently bonded, they have the highest melting and boiling point. For example, the melting point of Diamond is above 3500 °C which is the highest among all. Equally important, they are also hard and brittle. Some of the examples of Covalent network crystals are:
- Diamond (C)
- Quartz
- Boron (B)
- Silicon Dioxide (SiO2), etc.
Must Read: Ionic Bond Definition, Properties, Examples & Uses
Properties of Crystalline Solids
There are so many essential properties of crystalline solids. These are:
- Crystalline solids have well-defined edges and faces.
- They have a long-range of orders.
- They have a sharp melting point.
- Crystalline solids have definite heat of fusion.
- They are highly rigid and totally incompressible.
- They are anisotropic and symmetrical in nature, etc.
Examples of Crystalline Solids
If you think you can’t relate to crystalline solid examples in everyday life. Well, here is your chance to think again!
- Diamond
- Table salt
- Calcium fluoride
- Quartz
- Silicon dioxide
- Boron
- Gold
- Ammonia
- Iodine, etc.
Highly Recommended: Four Fundamental States Of Matter Explained
Applications of Crystalline Solids
There are so many essential applications of crystalline solids such as:
- Diamond is used for making jewelry.
- They are also used for cutting glasses.
- We use table salt in our food preparation.
- We use Quartz for making watches and wall clocks, etc.
Editor’s Choice: Ionic Bond vs Covalent Bond – Difference and Comparision
That’s it for this post. If you like this article, share it if you like, like it if you share it. You can also find us on Mix, Twitter, Pinterest, and Facebook. Hey man, If you have come this far, do give us feedback in the comment section. It would make my day. You can also make a donation. Your donations will help us to run our website and serve you BETTER. Cheers!!!

This was extremely helpful. Thank you very much!!
Thank you so much for your warm response, Michelle. Keep visiting us!!!