Whatever is around you is made up of matter. In general, there are three types of matter. These are solid, liquid, and gas. A solid can be further classified into three different groups. These are Crystalline Solids, Polycrystalline Solids, and Amorphous Solids. In this exclusive article, I am going to explain the definition of Amorphous Solid.
When molecules, atoms, or subatomic particles lack the long-range order, we call this type of solid an Amorphous solid. In other words, Amorphous Solids have highly irregular arrangements within their constituent particles.
Editor’s Choice: Difference Between Crystalline and Amorphous Solid
What is Amorphous Solid?
According to the definition of amorphous solids, a material whose molecules, atoms, or even sub-atomic particles lack the long-range order is known as amorphous solids. In other words, you can say that amorphous solids have irregular arrangements within their constituent particles. That’s why we also call them highly disordered solids or pseudo solids.
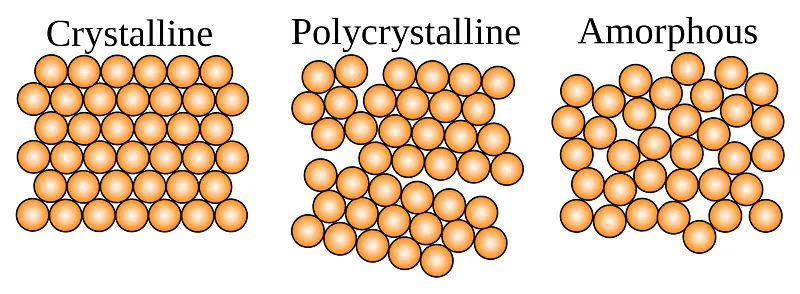
Additionally, the word amorphous comes from the ancient Greek word “morphé” which simply means formless. Just because the intermolecular distance between the two constituent particles always tends to vary. As a result, the intermolecular forces between them are never equal.
That’s why amorphous solids tend to soften over a wide range of temperatures rather than melting at sharp temperatures. This characteristic is one of the most important properties of amorphous solids.
Related: Crystalline Solid – Definition, Types, Properties & Examples
Properties of Amorphous Solids
There are so many essential properties of amorphous solids. These are:
- They have a short range of orders.
- They do not have a sharp melting point.
- Amorphous solids do not have definite heat of fusion.
- They are highly rigid but can be compressed.
- They are isotropic and unsymmetrical in nature.
- Amorphous solids do not have well-defined edges and faces, etc.
Editor’s Choice: Ionic Bond vs Covalent Bond – Difference and Comparision
Examples of Amorphous Solids
If you think you can’t relate to Amorphous solid examples in everyday life. Well, here is your chance to think again!
- Glass
- Wax
- Thin-film lubricants
- Gels
- Plastics
- Rubber
- Polymers, etc.
Highly Recommended: Four Fundamental States Of Matter Explained
Applications of Amorphous Solids
There are so many essential applications of amorphous solids in our day-to-day life. These are:
- We use plastics to make toys for kids.
- We use rubber to make tyres or sole of the shoes.
- Glass has been extensively used for making windows.
- We use amorphous solid in medical applications.
- We use amorphous solids in industrial applications, etc.
Check Out: Covalent Bond – Definition, Types, Properties & Examples
That’s it for this post. If you like this article, share it if you like, like it if you share it. You can also find us on Mix, Twitter, Pinterest, and Facebook. Hey man, If you have come this far, do give us feedback in the comment section. It would make my day. You can also make a donation. Your donations will help us to run our website and serve you BETTER. Cheers!!!
