By definition, a Covalent bond is a type of chemical bond that occurs due to the sharing of electrons between the participating atoms. Not to mention, these types of chemical bonds can only occur between non-metallic elements having the same or almost equal electronegativity values.
Additionally, the participating pairs of electrons in covalent bonding are known as shared pairs or simply bonding pairs. Based on the number of shared pairs of electrons, we can further bifurcate covalent further into further sub-groups. These are single, double, and triple covalent bonds.
Editor’s Choice: Difference Between Ionic and Covalent Bonds in Tabular Form
Examples of Covalent Bonds in Real Life
- Hydrochloric Acid (HCL)
- Oxygen (O2)
- Water (H2O)
- Cyanide (CN–)
- Carbon Dioxide (CO2)
- Nitrogen (N2)
Hydrochloric Acid (HCL)
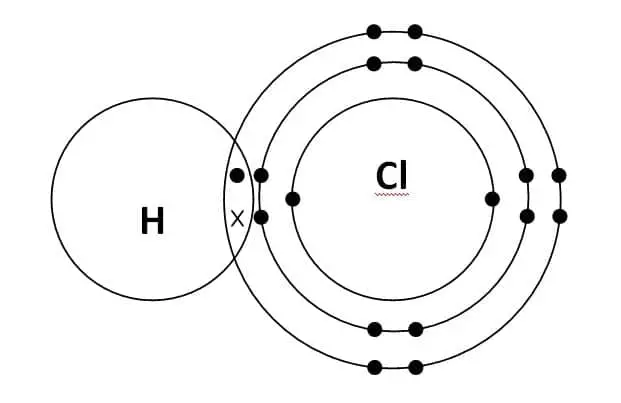
Hydrochloric Acid is nothing but an aqueous solution of hydrogen chloride that is a vital laboratory reagent and industrial chemical. A typical molecule of HCL consists of one hydrogen and one chlorine atom.
Hydrogen (H) has one valence electron in its outermost shell. Therefore, as per the octet rule, it requires one more electron to fill its orbital. Similarly, Chlorine (Cl) has seven valence electrons in its outermost shell. Hence, it also requires one more to fill up its orbital.
So, as per the laws of covalent bonding, in order to fill up the Octet of chlorine, hydrogen shares its lone electron with the chlorine atom. Similarly, in order to fill up the Octet of hydrogen, chlorine shares one electron with a hydrogen atom. Hence, forming a single covalent bond i.e sharing of two electrons.
Oxygen (O2)
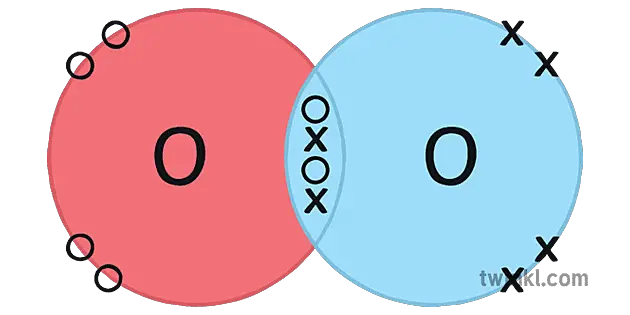
All the living beings, be it a human, a cat, or fish that resides underwater are living just because we have oxygen on our planet. As per the available data, 21 percent of the earth’s atmosphere is composed of oxygen. Not to mention, oxygen is the third most abundant element present in our observable universe.
A typical molecule of oxygen (O2) consists of two oxygen atoms. An oxygen atom has six valence electrons in its outermost shell. Hence, as per the Octet rule, it requires two more electrons to fill its orbital.
As per the law of covalent bonding, when two atoms of oxygen (O) combine to form an oxygen molecule (O2), they both share two electrons with each other to fill each other’s outermost shell. Hence, forming a double covalent bond i.e sharing of four electrons.
Water (H2O)
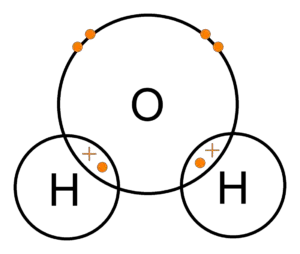
Just like oxygen, water is one of the most essential for the survival of life on planet earth. Water is used everywhere. Whether you are cooking or cleaning, you need water. There are so many industrial applications of water. To sum up, everything you eat, drink, and consume, is somehow related to the use of water.
A typical molecule of water (H2O)consists of two hydrogen atoms and one oxygen atom. Hydrogen (H) has one valence electron in its outermost shell. Hence, requires one more electron to fill its orbital. Similarly, oxygen (O) has six valence electrons in its outermost shell. Hence, requires two more electrons to fill its orbital.
As per the law of covalent bonding, in order to fill up the octet of two hydrogen atoms, oxygen shares one electron each with both of the hydrogen atoms. On the other hand, in order to fill up the octet of oxygen, both the hydrogen atoms share their lone electron with an oxygen atom. Hence, forming two single covalent bonds.
Cyanide (CN–)
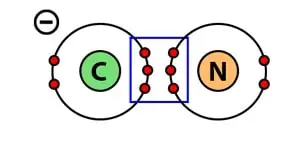
Cyanide is a chemical compound that we primarily use in plastic, paper, and textile industries. In fact, it is also used to develop photographs for you. A typical molecule of Cyanide (CN–) consists of one carbon atom and one nitrogen atom.
Carbon (C) has four 4 valence electrons in its outermost shell. Therefore, it requires four more electrons to fill up its orbital. On the other hand, nitrogen (N) has five valence electrons in its outermost shell. Therefore, it requires three more to fill up its orbital.
As per the law of covalent bonding, in order to fill its octet, when one atom of carbon and one atom of nitrogen combine to form Cyanide, both of them share three electrons with each other to fill their respective octet.
As a result, forming a triple covalent bond i.e sharing of six electrons. Not to mention, an extra available electron on carbon makes Cyanide an anion.
Editor’s Choice: Anion vs Cation – What’s the Difference??
Carbon Dioxide (CO2)

There are so many uses of carbon dioxide that we see in our day-to-day life. For example, they are used in fire extinguishers, carbonated beverages, refrigerants, etc. A typical molecule of carbon dioxide (CO2) consists one one carbon atom and two oxygen atoms.
Carbon (C) has 4 valence electrons in its outermost shell. Therefore, it requires 4 more to fill up its orbital. On the other hand, both of the oxygen (O) atoms have six valence electrons in their outermost shell. Therefore, they both require two-two more to fill up their orbital.
When one atom of carbon and two atoms of oxygen combine to form a chemical bond, in order to fill up the Octet of carbon, both of the oxygen atoms will share two electrons each with the carbon atom. Similarly, in order to fill up the Octet of both of the oxygen atoms, carbon will share two electrons each with the oxygen atoms. Hence, forming two double covalent bonds.
Nitrogen (N2)
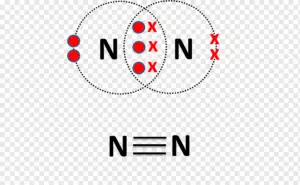
Nitrogen is used everywhere. As per the available data, a human body consists of approximately 3 percent of nitrogen by mass. In fact, they are the seventh most abundant element present in our observable universe.
A typical nitrogen molecule (N2) consists of two nitrogen atoms. An atom of nitrogen (N) has five valence electrons in its outermost shell. Therefore, requires three more electrons to fill its orbital.
So when two nitrogen atoms combine to form a nitrogen molecule, both of them share three electrons with each other. Hence, forming a triple covalent bond i.e sharing of 6 electrons.
Editor’s Choice: Difference Between Atom and Molecule in Table Form
Some other Examples of Covalent Bond in Everyday Life:
Apart from the above-mentioned ones, I am also mentioning some of a few here.
- Ethyne (C2H2)
- Ammonia (NH3)
- Methane (CH4)
- Carbon Monoxide (CO)
- Ethene (C2H4)
- Chlorine Molecule, etc.
That’s it for this post. If you like this article, share it if you like, like it if you share it. You can also find us on Mix, Twitter, Pinterest, and Facebook. Hey man, If you have come this far, do give us feedback in the comment section. It would make my day. You can also make a donation. Your donations will help us to run our website and serve you BETTER. Cheers!!!
You might also like:
- Covalent Bond – Definition, Types, Properties & Examples
- Ionic Bond Definition, Properties, Examples & Uses
- Crystalline Solid Definition, Types, Properties & Examples
- Amorphous Solid – Definition, Properties & Examples
